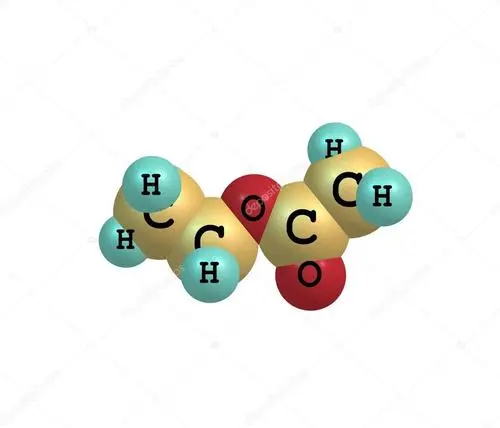
Ethyl acetate, also known as ethyl acetate, with chemical formula C4H8O2 and molecular weight 88.
11, is an ester with functional group -COOR (double bond between carbon and oxygen), which can undergo common reactions of general esters such as alcoholysis,
ammonolysis, ester exchange and reduction. Low toxicity, sweet taste, irritating odor at higher concentration, easy to volatilize, excellent solubility, fast drying, widely used, an important organic chemical raw material and industrial solvent.

It is a class I flammable product and should be stored in a low-temperature ventilated place, away from fire and ignition sources.
It is usually produced in the laboratory by esterification reaction between acetic acid and ethanol.
1. Chemical properties of ethyl acetate
Ethyl acetate, also known as ethyl acetate, is an ester with the functional group -COOR (a double bond between carbon and oxygen).
Ethyl acetate can also undergo alcoholysis, ammonolysis, ester exchange, reduction and other reactions common to esters in general.
Self-condensation in the presence of sodium metal produces 3-hydroxy-2-butanone or ethyl acetoacetate;
reaction with Grignard’s reagent produces ketones, which further react to give tertiary alcohols. Ethyl acetate is relatively stable to heat, no change when heated at 290℃ for 8~10 hours
. It decomposes into ethylene and acetic acid when passing through red-hot iron tube, decomposes into hydrogen, carbon monoxide, carbon dioxide
, acetone and ethylene through zinc powder heated to 300~350℃, and can decompose into water, ethylene, carbon dioxide and acetone through dehydrated alumina at 360℃. Ethyl acetate is decomposed by UV irradiation to produce 55% carbon monoxide,
14% carbon dioxide and 31% hydrogen or methane and other flammable gases. Reaction with ozone produces acetaldehyde and acetic acid. Gaseous hydrogen halide reacts with ethyl acetate to produce ethane halide and acetic acid.
Hydrogen iodide is the most reactive, while hydrogen chloride requires pressure to decompose at room temperature,
and is heated to 150°C with phosphorus pentachloride to produce ethyl chloride and acetyl chloride.
Ethyl acetate produces various crystalline complexes with metal salts. These complexes are soluble in anhydrous ethanol but not in ethyl acetate, and are readily hydrolyzed in water.
2. Main uses of ethyl acetate
1. GB 2760-1996 provides for the use of edible spices allowed.
Can be used in small quantities for magnolia, ylang-ylang, osmanthus, rabbit ear grass flowers and floral dew, fruit flavors and other fragrances for the first fragrance to bring up the fresh fruit fragrance, especially for perfume flavors,
with the effect of round ripe. Suitable for cherry, peach, apricot, grape, strawberry, hanging hook, banana, raw pear, pineapple, lemon, melon and other edible flavors.
Wine flavors such as brandy, whiskey, rum, yellow wine, white wine, etc. are also used.
2. Ethyl acetate is one of the most widely used fatty acid esters, it is a fast-drying solvent with excellent solvency ability, it is an excellent industrial solvent and can also be used as an eluent for column chromatography.
It can be used in nitrocellulose, ethylcellulose, chlorinated rubber and vinyl resin, cellulose acetate, butyl cellulose acetate and synthetic rubber, and also used in liquid nitrocellulose ink for copiers.
It can be used as a solvent for adhesive and a thinner for spray paint.
Ethyl acetate is an efficient solvent for many types of resins and is widely used in the production of ink and artificial leather.
It is used as analytical reagent, chromatographic analysis standard and solvent.
3. Toxicological information of ethyl acetate
Toxicity
It belongs to low toxicity category.
Acute toxicity: LD505620mg/kg (rat by mouth);
4940mg/kg (rabbit by mouth); LC505760mg/m3 for 8 hours (rat by inhalation);
2000ppm by human inhalation × 60 minutes, serious toxic reaction;
800ppm by human inhalation, with sickness; 400ppm by human inhalation for a short time, with irritation of eyes, nose and throat.
Sub-acute and chronic toxicity: guinea pigs inhaled 2000ppm, or 7.2g/m3 amount, 65 times exposure, no significant effect; rabbits inhaled 16000mg/m3×1hour/day×40 days,
anemia, increased white blood cells, organ edema and fatty degeneration.
4. Storage and transportation of ethyl acetate
1、This product is a first-class flammable product, should be stored in a low-temperature ventilation, away from fire and fire sources.
2、Take measures to prevent the occurrence of static electricity. When loading and unloading,
it should be lightly loaded and unloaded to prevent the packaging and container from being broken and to prevent the accumulation of static electricity.
3, the product should be stored in a cool, ventilated warehouse, the warehouse temperature should not exceed 30 ℃, prevent direct sunlight, keep the container closed.
It should be stored separately from oxidizers, acids and bases, etc.

The storage area should be equipped with leakage emergency equipment and suitable shelter materials. Prohibit the use of spark-prone machinery and tools.
4, the workplace should be kept ventilated and breathable, the operator should wear good protective equipment
5. Ethyl acetate safety measures
Hazardous characteristics
Flammable, its vapor and air can form an explosive mixture.
When exposed to open flame and high heat, it can cause combustion and explosion. Reacts violently when in contact with oxidizer.
In the fire, there is a risk of explosion in the heated container.
Its vapor is heavier than air, can be spread to a considerable distance at a lower place, in contact with an open flame will lead to back ignition.
Combustion (decomposition) products: carbon monoxide, carbon dioxide.
Field emergency monitoring method: gas detection tube method.
Laboratory monitoring methods: pumpless sampling gas chromatography (WS/T155-1999, workplace air)
Vapors may cause drowsiness and vertigo. Long-term exposure may cause dry and cracked skin
Emergency treatment
Inhalation:
Quickly remove from the scene to fresh air. Keep the airway open.
If breathing is difficult, give oxygen infusion. If breathing stops, give artificial respiration immediately. Seek medical attention.
Accidental ingestion: Drink enough warm water, induce vomiting, seek medical attention.

Skin contact: Remove contaminated clothing and rinse skin thoroughly with soapy water and water.
Eye contact: Lift eyelids and flush with running water or saline. Seek medical attention.
Extinguishing agents: Soluble foam, carbon dioxide, dry powder, sand and earth. Extinguishing with water is not effective.
Fire extinguishing precautions: Water may be used to keep containers in the fire cool.
Spill handling
Quickly evacuate people from the contaminated area to a safe area, isolate and strictly limit access. Cut off the source of fire.
It is recommended that emergency personnel wear self-contained positive pressure respirators and fire protective clothing.
Cut off the source of the spill as much as possible and prevent access to restricted spaces such as sewers and flood drains.

Small spills: Absorb with activated carbon or other inert materials.
It can also be flushed with large amounts of water and diluted with wash water and put into the wastewater system.
Large spills: Construct a dike or dig a pit to take in; cover with foam to reduce vapor disaster.
Transfer to tanker or special collector with explosion-proof pump, recycle or transport to waste disposal site for disposal.
Contraindications for compatibility
Ethyl acetate reacts violently with strong oxidizing agents, strong bases, strong acids and nitrates, which may lead to fire or explosion.
It also reacts violently with chlorosulfonic acid, lithium aluminum hydride, 2-chloromethylfuran, and tetrabutylamine hydroxide.
Alarm Detection
Combustible gas concentration alarms are used to detect leaks of combustible gases.
When there is a combustible gas leak in the industrial environment, when the gas concentration alarm detects that the gas concentration reaches the explosion threshold, t
he combustible gas concentration alarm will issue an alarm signal to remind the site staff to take safety measures and drive the exhaust, cut off,

spraying system to prevent the occurrence of explosion, fire, poisoning accidents, so as to ensure safe production.
6. Ethyl acetate regulatory information
Ethyl acetate (summer embargo)
The product is included in the “First List of Key Regulated Hazardous Chemicals
Ethyl acetate is a flash point flammable liquid in category 3.2
Intrusion route: inhalation, ingestion, transdermal absorption
7. Safety Instructions for Ethyl Acetate
Ethyl acetate is low in toxicity.

Health hazards: Irritating to eyes, nose and throat.
High concentration inhalation can lead to progressive anesthesia, acute pulmonary edema, liver and kidney damage. Continued inhalation of large amounts can cause respiratory paralysis.
Accidental ingestion can cause nausea, vomiting, abdominal pain, diarrhea, etc. Allergenic effect, gum bleeding due to vascular nerve disorder; can cause eczema-like dermatitis.
Chronic effects: Long-term exposure to this product can sometimes lead to corneal clouding, secondary anemia, leukocytosis, etc.
Flammability hazard: This product is flammable, irritating and allergenic.
Hazardous characteristics: flammable, its vapor and air can form an explosive mixture, in contact with open fire, high heat can cause combustion and explosion.
Reacts violently when in contact with oxidizer. Its vapor is heavier than air, can be spread to a considerable distance at a lower place, and will catch fire when it meets an ignition source.

 By Coco Ho
By Coco Ho