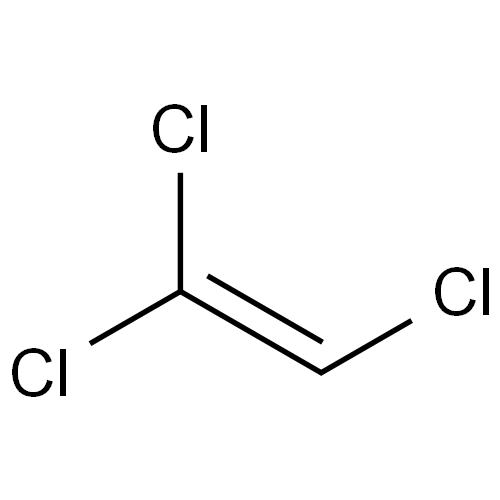
Trichloroethylene, chemical formula C2HCl3, a compound produced by replacing three hydrogen atoms in the ethylene molecule with chlorine.
It is insoluble in water and soluble in ethanol, ether, etc. Trichloroethylene is a flammable liquid and can cause fire and explosion when exposed to open flame and high heat.
Trichloroethylene has been used as an analgesic and metal degreaser, as an extractant, fungicide and refrigerant, and as a dry cleaning agent for clothes.
Long-term exposure can cause conditions such as trigeminal nerve palsy.
On October 27, 2017, the World Health Organization’s International Agency for Research on Cancer published a preliminary compilation of references to the list of carcinogens,
and trichloroethylene is on the list of Class I carcinogens. On July 23, 2019, trichloroethylene was added to the list of toxic and hazardous water pollutants (first batch).
1. Chemical properties of trichloroethylene
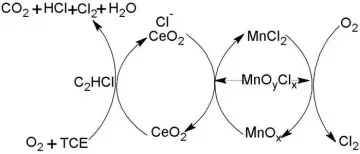
It has no corrosive effect on general metals below 120℃. [5] React with 90% sulfuric acid to form monochloroacetic acid;
add with chlorine to form pentachloroethane. [6] Reacts with oxygen when heated or at high temperature to form highly toxic phosgene.
2.Trichloroethylene action and use
Excellent solvent, used as metal surface treatment agent, cleaning agent before electroplating and painting, metal degreasing agent and extractant of fat,
oil and paraffin. It is used in organic synthesis and pesticide production. Trichloroethylene is used in the production of tetrachloroethylene as an anthelmintic;
it is used in the production of hexachloroethane as a veterinary anthelmintic for the control of liver leech disease and gastric leech disease,

testicular schistosomiasis and nematode disease in ruminant animals.
3. Properties and stability of trichloroethylene
1. Chemical properties: Trichloroethylene without stabilizer is gradually oxidized in air to produce phosgene, carbon monoxide and hydrogen chloride.
A small amount of dimer (hexachlorobutene) may also be produced. The reaction proceeds on a free-radical course and is significantly promoted by light and heat.
In the presence of moisture, dichloroacetyl chloride decomposes to dichloroacetate and hydrogen chloride.
The acid produced by decomposition corrodes metals.
Therefore, trichloroethylene is usually used in industry with the addition of trace amounts of stabilizers such as phenols (hydroquinone), amines or alcohols.
In the presence of air, moisture and light, trichloroethylene with added stabilizers does not work with general industrial metal materials even when heated to 130°C.
2. When trichloroethylene vapor is heated above 700°C, it decomposes to form a mixture of dichloroethylene, tetrachloroethylene, carbon tetrachloride, chloroform, and chloromethane.
When trichloroethylene vapor is subjected to strong air, it is completely oxidized to produce carbon dioxide, hydrogen chloride, carbon monoxide and phosgene.
In the presence of copper salts, trichloroethylene is heated at 175°C under pressure and reacts with aqueous solutions or suspensions of hydroxides of alkali metals or alkaline earth metals to produce hydroxyacetic acid salts.

When cold, it does not react with hydrochloric acid and nitric acid, but when heated, it reacts violently with concentrated nitric acid and decomposes completely,
and trichloronitromethane and monochlorodinitromethane can be obtained by controlling the reaction conditions.
Under the catalytic effect of aluminum trichloride, it reacts with hydrogen chloride at 30~50℃ to produce 1,1,1,2-tetrachloroethane.
In the presence of caustic alkali easily dechlorinated hydrogen reaction to generate dichloroacetylene, dichloroacetylene in the air spontaneous combustion and explosive decomposition.
Sodium carbonate and liquid ammonia do not react with trichloroethylene under normal conditions. Aluminum metal,
especially powdered aluminum metal, can cause the decomposition of trichloroethylene without stabilizer, generating hydrogen chloride and at the same time,
strong explosive decomposition or carbonization. The reaction is initiated into aluminum trichloride,

which acts as a Friedel Crafts catalyst to induce a condensation reaction of trichloroethylene to produce pentachlorobutadiene,
which is further condensed into resins and tar. In the presence of aluminum trichloride, trichloroethylene reacts with chloroform to form 1,1,1,2,3,3-hexachloropropane.
Reacts with carbon tetrachloride to form 1,1,1,2,3,3,3-heptachloropropane. Heating under pressure to 150-200°C in the presence of a peroxide, e.g.
benzoyl peroxide, yields dimer and trimer of trichloroethylene. It is readily chlorinated under the catalysis of ferric chloride to form pentachloroethane and hexachloroethane.
3. Stability: stable

4. Prohibited substances: strong oxidizing agent, strong reducing agent, strong alkali, aluminum, magnesium
5. Avoid contact conditions: light, ultraviolet light
6. Polymerization hazard: polymerization
7. Decomposition products: hydrogen chloride
4. Precautions for use
Summary of Hazards
Health hazard: The product has a narcotic effect mainly on the central nervous system. It may also cause liver, kidney, heart and trigeminal nerve damage.
Acute poisoning: Exposure (inhalation, percutaneous or oral) to large amounts of this product in a short period of time can cause acute poisoning. Inhalation of very high concentrations can lead to rapid coma.
Eye and upper respiratory tract irritation may occur after inhalation at high concentrations.
After a few hours of exposure, headache, dizziness, drowsiness and drowsiness may occur, and in severe cases, delirium, convulsions, coma, respiratory paralysis and circulatory failure may occur.
Cranial nerve damage, mainly trigeminal nerve damage, and cardiac damage, mainly cardiac arrhythmia, may occur.
There may be liver and kidney damage. Oral gastrointestinal symptoms are obvious, and liver and kidney damage is prominent.
Chronic toxicity: still controversial.
Headache, dizziness, weakness, sleep disorders, gastrointestinal disorders, peripheral neuritis, myocardial damage, trigeminal nerve palsy and liver damage occur. May cause skin damage.
Environmental hazard: It is a serious hazard to the environment and can cause pollution to water bodies and the atmosphere.
Fire and explosion hazard: the product is flammable, toxic and irritating.
First aid measures
Skin contact: Remove contaminated clothing immediately and rinse skin thoroughly with soapy water and water. Seek medical attention.
EYE CONTACT: Lift eyelids and flush with running water or saline. Seek medical attention.
Inhalation: Quickly remove from the scene to fresh air. Keep airway open. If breathing is difficult, give oxygen infusion.
If breathing stops, give immediate artificial respiration. Seek medical attention.
Ingestion: Drink sufficient warm water and induce vomiting. Seek medical attention.
Fire-fighting measures

Hazardous characteristics: In contact with open flame and high heat can cause combustion and explosion.
Contact with strong oxidizing agents can lead to chemical reaction.
Exposure to ultraviolet light or decomposition during combustion or heating produces toxic phosgene and corrosive hydrochloric acid fumes.
Harmful combustion products: carbon monoxide, carbon dioxide, hydrogen chloride, phosgene.
Fire fighting methods: Firefighters must wear oxygen breathing apparatus.
Spray water to keep the fire container cool until the end of the fire.
Extinguishing agents: fog water, foam, dry powder, carbon dioxide, sand.
Leak emergency treatment
Emergency response: Quickly evacuate people from the contaminated area of the spill to a safe area, and isolate and strictly limit access.
Cut off the source of fire. Suggest that emergency personnel wear self-contained positive pressure respirators and hazmat suits.
Cut off the source of the spill if possible. Prevent flow into restricted spaces such as sewers and flood drains.
Small spills: Adsorb or absorb with sand or other non-combustible materials.
Large spill: Construct a dike or dig a pit to shelter it. Cover with foam to reduce vapor disaster.
Transfer to a tanker or special collector with a pump, recycle or transport to a waste disposal site for disposal.
Operation disposal and storage

Operation precautions: Airtight operation and enhanced ventilation.
Operators must be specially trained and strictly follow the operating procedures. It is recommended that operators wear self-absorbing filtered gas masks (half masks),
chemical safety protective glasses, anti-toxicant penetration overalls and chemical resistant gloves.
Keep away from fire and heat sources, and smoking is strictly prohibited in the workplace. Use explosion-proof ventilation systems and equipment.
Prevent vapors from leaking into the workplace air. Avoid contact with oxidizers, reducing agents, alkalis, and metal powders.
Handling should be lightly loaded and unloaded to prevent damage to packaging and containers.
Equipped with the appropriate variety and quantity of fire-fighting equipment and leak emergency handling equipment.
Empty containers may have residual harmful substances.

Storage precautions: Store in a cool, ventilated warehouse. Keep away from fire and heat sources. S
torage temperature should not exceed 25℃ and relative humidity should not exceed 75%. Packaging requirements are sealed and should not be in contact with air.
It should be stored separately from oxidizers, reducing agents, alkalis, metal powders and edible chemicals, and should not be mixed.
It should not be stored in large quantities or for a long time. Equipped with corresponding varieties and quantities of fire-fighting equipment.
The storage area should be equipped with emergency treatment equipment for leaks and suitable shelter materials.

 By Coco Ho
By Coco Ho